Lithium Chloride Evaporation System
Industrial Evaporation Solutions
We provide LiCl evaporation solutions for producing lithium chloride anhydrous used in air-conditioning, welding and soldering flux and desiccants
Anhydrous lithium chloride is commonly used in the production of lithium metal through electrolysis. It also serves as a flux in aluminum welding and brazing, and as a desiccant in non-refrigerant air conditioning systems due to its strong moisture absorption capacity.
In industrial applications, lithium chloride is typically obtained from lepidolite, spodumene, or from brine solutions after the extraction of sodium chloride and potassium chloride. Evaporation of lithium chloride solution produces lithium chloride monohydrate crystals, while heating above 98 °C results in the formation of anhydrous lithium chloride.
| Temperature (°C) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
| Solubility (g/100 g H₂O) | 69.2 | 74.5 | 83.5 | 86.2 | 89.8 | 93.6 | 98.4 | 10.7 | 112 | 121 | 128 |
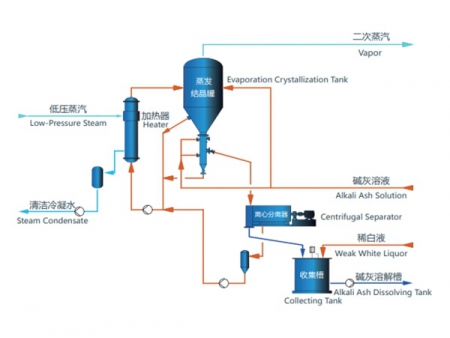
When lithium chloride is produced from spodumene, the resulting solution, after reaction, precipitation, filtration, and acidification, contains very low levels of sodium chloride. Similarly, brine-based lithium salt production often involves impurities such as sodium and potassium chlorides. To ensure high-purity lithium chloride, sodium and potassium must be separated efficiently during evaporation and concentration, with minimal lithium loss during separation. The crystallization process also presents challenges such as pipe blockages and foam generation, which can lead to lithium being carried over and lost. Based on material analysis and proven performance in industrial applications, we have made specific design improvements to evaporation equipment in the production of lithium chloride from lithium-rich solutions. These modifications enhance process stability and help reduce losses.
Due to the high boiling point elevation of lithium chloride solutions, triple-effect evaporators are used for concentration. As the solution becomes more concentrated, sodium chloride crystals begin to precipitate due to the common ion effect. The resulting mixture is filtered to obtain a purified lithium chloride concentrate, which is then processed using forced circulation crystallizers to produce anhydrous lithium chloride.
We provide comprehensive evaporation and crystallization solutions for various applications, including:
- Lithium chloride evaporation and crystallization in spodumene-based processes
- Sodium and potassium removal from lithium chloride solutions in brine-based extraction
- Recovery of lithium chloride from lepidolite processing
- Lithium chloride recovery systems for PPS (polyphenylene sulfide) production
- Other lithium chloride recovery needs across multiple industries
- High sodium and potassium removal efficiency is achieved during evaporation, with minimal lithium loss carried over alongside these impurities.
- The concentration system uses a combination of different evaporator types, optimized to reduce both operational costs and upfront equipment investment while maintaining process stability.
- The crystallizer is carefully designed to minimize lithium loss caused by mist entrainment during evaporation and to support proper crystal formation and growth.