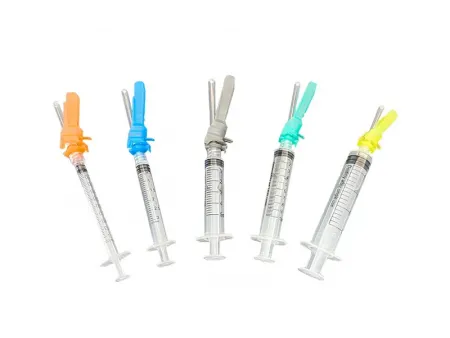
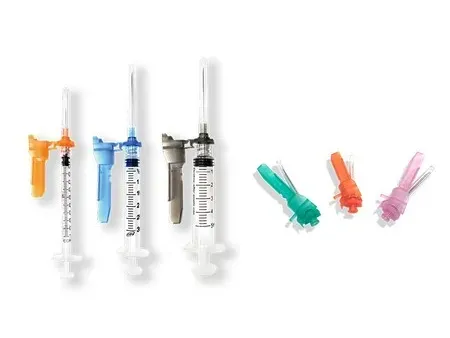
Safety Syringe
Request a Quote
Single-use Disposable Medical Products Manufacturer Since 1997
Hypodermic Supplies
- Approval: US-FDA 510(k),EU-MDR and ISO13485
- Sterilization Method: ethylene oxide sterilization, non-toxic, non-pyrogenic
- Syringe Components: syringe barrel, plunger rod and stopper (piston)
- Syringe Volume: 1ml, 1.2ml, 2mL, 2.5mL, 3 ml, 5ml, 6ml, 10ml, 20ml, 30ml, 50ml, 60ml, and 100ml
- Needle Components: needle hub, needle shaft and pivoting shield
- Needle Gauge: 27G, 26G, 25G, 24G, 23G, 22G, 21G, 20G, 19G, and 18G
Registration Number of Approval Certificates
- US FDA 510 (k) No.: K053519
- EU MDR No.: G10 045084 0043 Rev.00
- EU ISO13485 No.: Q5 045084 0039 Rev.01
Download Registration Certificate
Safety syringes are designed for subcutaneous, intramuscular, and intravenous injections, as well as for blood draws and medication preparation. This medical needle significantly reduces the risk of blood-borne diseases, particularly HIV and hepatitis.
Features
- Allows for accurate control of liquid volume, preventing wastage and adverse reactions due to overdosage
- The syringe is designed to leave minimal residual liquid, with moderate pressure to accommodate various injection methods
- Compatible with different needle sizes to meet various injection needs
Packaging
Our safety syringes are carefully packaged to ensure sterility and protection:
- Individual packaging: Sterile packed per piece in blister packaging
- Intermediate packaging: Individually packed syringes are grouped into boxes of 100 units
- Bulk packaging: These boxes are then placed into large, durable corrugated cardboard cartons for safe transport and storage