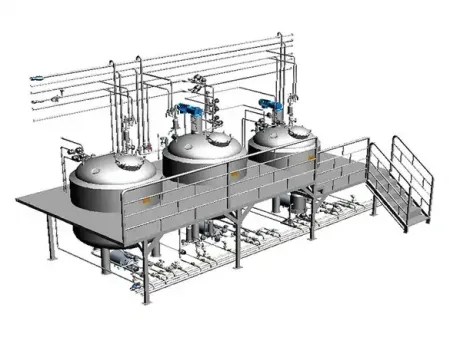
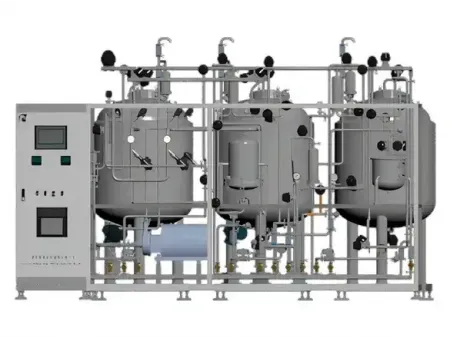
Pharmaceutical Liquid Preparation System
We offer integrated Pharmaceutical Liquid Preparation Systems designed for the preparation of sterile injectables, monoclonal antibodies, vaccines, and plasma-derived products. Each Pharmaceutical Liquid Preparation System is customized to meet on-site layout requirements, preferred cleaning and sterilization protocols, and international pharmaceutical standards.
Our formulation tanks and preparation vessels are engineered to ensure homogeneous mixing and temperature stability during the entire compounding process. The system layout is planned for optimal space efficiency and easy access, with smooth internal surfaces and clean piping connections to eliminate dead legs and contamination risks.
Complying with GMP, ISPE guidelines, and FDA regulations, our pharmaceutical Pharmaceutical Liquid Preparation Systems include hygienic piping, automated process control, and support for complete qualification documentation (DQ, IQ, OQ, PQ). From design to installation and validation, WEMAC ensures your production environment remains compliant and ready for global markets.
Widely used in the production of aseptic injectables, monoclonal antibodies, biological vaccines, and plasma-derived products.
- Insulation thickness: ﹥30 mm for stable temperature retention
- Surface roughness: Ra ﹤ 0.38 μm, easy to clean and hygienic
- Mixing speed: Adjustable from 50 to 2900 rpm
- Temperature range: Heats to 80°C or cools to 20°C within 30 minutes
- CIP/SIP time: Less than 1 hour for a full cycle
Note: Each pharmaceutical water system is custom-designed to meet the user requirement specification (URS) provided by the client. All photos and videos featured are from actual WEMAC projects.
- To reduce welding points and contamination risks, stainless steel tubing is bent directly whenever possible. When welding is needed, argon-shielded automatic welding is applied using single-side welding with double-side formation, followed by electro-polishing to ensure a clean, smooth surface.
- Validation materials include borescope inspection photos, weld maps, pressure test results, passivation reports, and complete design documentation for regulatory compliance.
- The control platform runs on a fully automated HMI and PLC system. It offers a simple user interface, supports various communication modes, and allows configuration of at least three user access levels.
- Supervisory control and data acquisition (SCADA) integration is available for real-time data collection and centralized monitoring, helping to maintain stable operation across all areas.
- Conductivity, temperature, and flow are tracked continuously with key data recorded via an onboard data logger to support traceability.
- Mixing tanks in separate rooms can be independently controlled and displayed on-screen, reducing manual handling and streamlining the production process.
- Concentrated formulation tank
- Diluted formulation tank
- In-line monitoring sensors
- Microporous filter (1–0.22 μm precision)
- Transfer pump
- Nitrogen-sealed piping
- Self-cleaning circulation pipeline
The automated CIP system simplifies the cleaning process with one-touch operation and occupies minimal floor space, making it suitable for mobile or compact setups.
Sampling valves support both CIP and SIP in-place. Temperature sensors at the drainage point enable automatic FO value recording when required. The spray ball is designed using a sanitary static spray ball for effective and hygienic cleaning.
Integrated with level and temperature sensors, the flange connection ensures zero dead legs and hygienic monitoring at the tank base.
Designed for aseptic sampling without leaving residues, this flush-mounted valve enables clean, dead-space-free operation.
Incorporates a load cell and diaphragm control valve to accurately control dosing and maintain consistent formulate ratios.
A hygienic drawbar-style outlet at the tank top minimizes cleaning blind spots, improving CIP coverage and overall sterility.