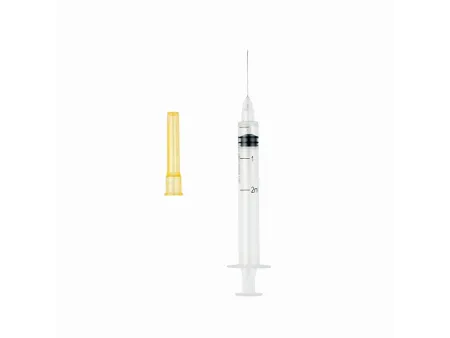
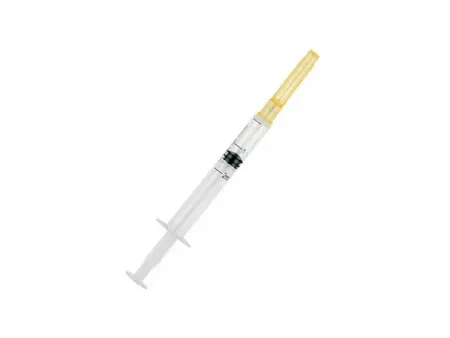
Auto-Disable Syringe (Breaking Plunger)
Request a Quote
Single-use Disposable Medical Products Manufacturer Since 1997
Hypodermic Syringe with Reuse Prevention Feature (RUP Syringe)
- Approval: EU-MDR and ISO13485, WHO certificate
- Sterilization Method: ethylene oxide sterilization, non-toxic, non-pyrogenic
- Syringe Volume: 1ml, 2ml, 3ml, 5ml, 10ml
Registration Number of Approval Certificates
- Jiangsu Medical Device Registration Certificate No.: 20173154044
- EU MDR No.: G10 045084 0043 Rev.00
- EU ISO13485 No.: Q5 045084 0039 Rev.01
- WHO (World Health Organization) Certificate No.: E013/041, E013/086, E013/133, E013/029, E013/083, E013/058, D013/073
- WHO PQS No.: E013/029, E013/041, E013/058, E013/073, E013/083, E013/086, E013/133, E013/136
Download Registration Certificates
The auto-disable syringe with a breaking plunger is designed for clinical use, including medication injections, blood draws, and fixed-dose vaccine injections over 1ml. This syringe type has a design aimed to enhance patient safety by preventing reuse, thereby reducing the risk of cross-contamination and infection.
Components
The auto-disable syringe comes in two versions: with and without a fixed needle. Both types feature a concentric tip and consist of three main parts, along with an additional auto-disable component.
- Fixed needle type: Includes an outer sleeve, plunger, piston, needle cover, needle tube, breaking plunger mechanism, and needle hub.
- Non-fixed needle type: Comprises an outer sleeve, plunger, piston, and breaking plunger mechanism, and is used with disposable sterile needles.
Features
- The syringe self-destructs after use without requiring any additional actions or changes in user operation, making it easy for healthcare professionals to use
- The auto-disable feature activates seamlessly upon completion of the injection, with no noticeable resistance, ensuring ease of use without the need for extra force
- The elastic stopper on the plunger prevents accidental activation before use
- The fixed needle design minimizes dead space, resulting in lower medication residue compared to standard syringes
- 5.After use, both the needle and syringe cannot be reused, ensuring maximum safety and hygiene
Packaging
To maintain the integrity and sterility of the auto-disable syringes, they are available in three packaging levels:
- Individual packaging: Each syringe is sterile packed in blister packs or high-pressure PE bags to ensure each unit is protected and ready for use.
- Intermediate packaging: The individually packed syringes are grouped into medium-sized boxes made from grey base whiteboard paper or fine corrugated materials. This level of packaging provides additional protection and facilitates handling.
- Bulk packaging: Finally, the medium boxes are placed into large, durable corrugated cardboard cartons. This bulk packaging method ensures robust protection during transport and storage, guaranteeing that the products remain in optimal condition until they reach their final destination.