BFS Vacuum Decay Leak Testing Machine
Container Closure Integrity Testing (CCIT)
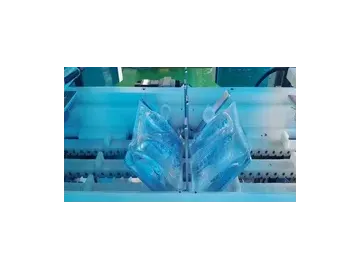
- Applicable container: BFS plastic ampoules (clean and dry)
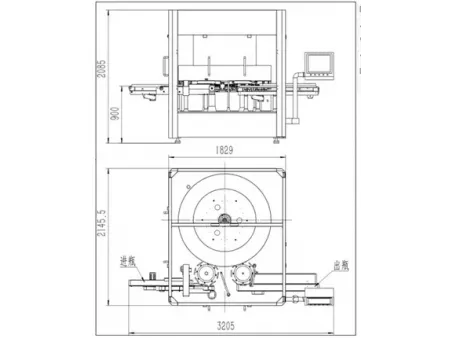
- Max. size of container: 135×900mm
- Machine dimensions (L×W×H): 3000×1900×2200mm
- Power supply: 380V 3 phase 50Hz
- Designed for non-destructive integrity testing of BFS plastic ampoule containers filled with pharmaceutical products
- Supports 100% online and offline inspection, even under high-speed production conditions
- Leak detection is performed inside a sealed test chamber with a pressure differential
- Determines container integrity by measuring achieved pressure levels and monitoring pressure changes during the test cycle
- Capable of identifying micro leaks, seal failures, and cracks that may compromise sterility
- Requires test containers to be clean and dry for accurate results
- Suitable for installation in Class C and D cleanroom environments during less critical aseptic processing stages
- All validation and qualification processes are fully aligned with current Good Manufacturing Practice (GMP) guidelines.
- Materials and construction methods meet the hygiene and quality standards required in pharmaceutical production environments.
- The testing system features a polycarbonate safety shield to prevent injuries from accidental contact or compression.
- Its clean design minimizes the risk of contamination by eliminating hard-to-clean corners or areas where residue might accumulate.
- A clear separation is maintained between the technical components and the product-handling zone to ensure controlled processing conditions.
- Automatic feeding and unloading streamline the handling of BFS containers during the testing process
- High sensitivity enables the detection of even the slightest leaks with precision
- Delivers fast, reliable, and repeatable test results for consistent quality assurance
- Utilizes a non-invasive, non-destructive method that preserves product integrity
- Enhanced HMI offers intuitive controls and smooth system interaction
- Built-in self-diagnostic capabilities improve operational reliability
- Hygienic design with no hidden corners simplifies cleaning procedures
- All moving parts are easily accessible, making routine maintenance more efficient
- Tracks and stores historical production, testing, event, and alarm data for traceability
- Real-time display of leak testing curves on the HMI helps operators monitor performance instantly
Vacuum-based leak testing is carried out using 30 chambers, each designed to perform non-destructive integrity testing on sealed pharmaceutical containers. Once capped, the containers are transferred via a conveyor, then spaced apart by a screw feed system and positioned beneath the inspection units. Each chamber lowers to enclose the bottle in a sealed, fixed-volume cavity.
Inside the chamber, the testing system measures vacuum levels and monitors any pressure changes over time. A pressure drop that exceeds the allowed threshold indicates a potential leak, such BFS containers are automatically removed from the line. Accepted products continue smoothly to the next stage.
Tailored for high-throughput production lines, this leak testing solution can be integrated directly into existing setups, helping maintain both speed and quality without compromising container integrity.