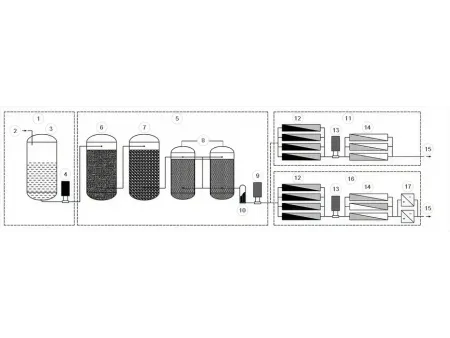
Purified Water System (PW Generation)
Pharmaceutical-grade purified water is produced in compliance with international pharmacopeia standards, including the Chinese Pharmacopoeia (CP), United States Pharmacopeia (USP), and European Pharmacopoeia (EP). These purified water systems typically rely on multi-stage filtration and membrane-based purification technologies, ensuring consistent water quality for drug production, laboratory use, and equipment rinsing. We offer complete system configurations tailored to specific user needs, covering everything from engineering design and equipment selection to automated control and validation support.
- Raw water
- Potable water
- Raw water tank
- Raw water pump
- Pretreatment
- Multimedia filter
- Activated carbon filter
- Duplex softener
- Primary pump
- Safety filter
- RO RO
- RO First-pass RO
- Inter-stage pump
- RO Second-pass RO
- Purified water
- RO RO EDI
- EDI unit
Our purified water production systems are designed according to the quality of the incoming water, required water standards, and the specific conditions of each project. WEMAC offers both customized and standardized solutions. Depending on the configuration, the pure water generation system can deliver water that complies with the quality specifications defined by the Chinese (CP 2020), United States (USP 43), and European (EP 10) Pharmacopoeias.
- Electrical Conductivity: ≤2μS/cm
- Electrical Resistivity: ≥0.5MΩ•cm
- Microbial Count: ≤10CFU/100ml
- Endotoxin: ≤0.25EU/ml
- Heavy Metal Content: ≤0.5μg/ml
- Ammonia: ≤0.3μg/ml
- pH Value: 6.5~8.5
Note: Each pharmaceutical water system is custom-designed to meet the user requirement specification (URS) provided by the client. All photos and videos featured are from actual WEMAC projects.
- Pharmaceutical manufacturing and formulation
- Laboratory research and quality control testing
- Sterilization and cleaning of medical instruments and equipment
- Biotechnology development and experimental processes
- Aseptic filling and packaging operations
- Cooling tower and HVAC system maintenance
- Any process requiring high-purity water
When the conductivity exceeds the predefined threshold, the purified water system redirects the water back to the intermediate tank to maintain water quality before entering the next stage.
If the conductivity remains within acceptable limits, the water is delayed and sent to the purified water storage tank, ensuring consistent quality without premature transfer.
Uses first-pass permeate to flush the reverse osmosis membranes. The intermediate tank acts as both the starting and return point for the cleaning cycle.
- Our purified water system is built using a modular approach, ensuring a compact structure and well-organized layout that makes daily operation and maintenance more straightforward.
- The dual softening tanks are connected through a smart valve group, allowing them to run in series or take turns regenerating. Each tank can supply 100% of the required flow, ensuring continuous operation with no downtime.
- Both the raw water pump and the two-stage high-pressure pumps are equipped with frequency converters. This allows precise pressure regulation depending on each operational phase, whether during filtration, softening, or standby mode. While idle, the pure water generation system enters a low-speed internal loop that helps minimize microbial growth and save energy.
- At the final stage, the purified water is sent through two independent pipelines into the storage tank when quality meets the standard. If quality does not meet the required level, the water recirculates back to the intermediate tank. When the purified water tank reaches full capacity, the system automatically switches to circulation mode, preventing dead legs and ensuring constant flow.
- Stainless steel piping is bent directly whenever possible, reducing welding points. For necessary joints, argon-shielded automatic welding is used (single-side weld with double-sided molding), ensuring weld integrity. All welds undergo post-processing with electrochemical polishing.
- Delivered with complete validation materials, including videos or photos from endoscopy inspections, weld maps, pressure test reports, pickling and passivation records, and full system design documentation.
- The process is fully automated using a PLC paired with a user-friendly HMI touchscreen. Various communication protocols are available, and access levels can be configured into at least three user roles for security and traceability.
- Real-time monitoring of conductivity, temperature, and flow rate is supported. Users can choose between paperless or paper-based recorders to log and print critical process parameters.
- The control system can be configured with electronic signatures and audit trail functionality to comply with GAMP 5 and FDA 21 CFR Part 11 requirements for regulated pharmaceutical operations.
Our purified water system includes a pretreatment unit, raw water storage tank, primary filtration section, and a purified water storage tank. Each section is integrated to ensure reliable water quality and smooth system operation from the initial stage to final output.
- A. Electrical control system
- B. Terminal water distribution design
- C. Internal cleaning cycle